【 Drug name 】
Generic name: Citalopram hydrobromide tablets
Product Name: Citalopram hydrobromide tablets (Mikewei)
English name: Citalopram Hydrobromide Tablets
Pinyin code: all QingXiuSuanXiTaiPuLanPian
[Main ingredients] Citalopram hydrobromide
【 component 】
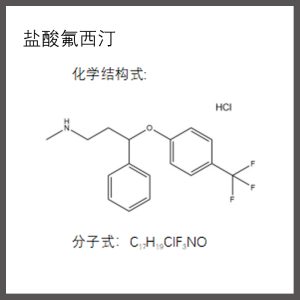
Chemical name: 1-(3-dimethylaminopropyl)-1-(4-fluorophenyl)-1, 3-dihydroisobenzofuran-5-nitrile, hydrobromide
Molecular weight: C20H22BrFN2O
[Nature] This product is a film coat, after removing the coating, it appears white or off-white.
[Indications/functions] for the treatment of depression.
[Specification Model] 20mg*10s(Mikewei)
[Usage and dosage] Adults: take once a day, 20mg each time. Depending on the individual patient’s response, the dose may be increased up to a maximum dose of 40mg daily. The duration of treatment is usually 2 to 4 weeks after the onset of antidepressant effects. “Antidepressant treatment is symptomatic and therefore must be continued for an appropriate length of time (usually up to 6 months after recovery) to prevent relapse.” In patients with recurrent depression, maintenance therapy may need to be continued for many years to prevent recurrent episodes. Older patients (>65) Older patients should have their dose reduced to half the recommended dose of 10 to 20 mg daily. The maximum recommended dose is 20 mg daily. Children and Adolescents (<18 years) This product is not indicated for children and adolescents under 18 years of age. See instructions for details.
[Adverse reactions] Adverse reactions to this product are usually transient and mild. It is usually evident within the first or second week after taking the drug and generally resolves as depressive symptoms improve. The common adverse reactions were nausea, dry mouth, dizziness, headache, somnolence, shortened sleep time, hyperhidrosis, decreased salivation, tremor, and diarrhea. Among the adverse reactions reported in foreign clinical studies and post-marketing, the dose-related adverse reactions included fatigue, impotence, insomnia, hyperhidrosis, somnolence, and yawning. “The following rare reports of adverse effects are temporarily considered to be related to this drug: angioedema, choathetosis, epidermal necrosis, erythema multiforme, hepatic necrosis, malignant antidepressant syndrome, pancreatitis, serotonin syndrome, spontaneous abortion, thrombocytopenia, arrhythmia, priapism, torsades de pointes, and withdrawal syndrome.” The following adverse events have also been found in clinical studies abroad, but they are not necessarily caused by this product. Cardiovascular system: tachycardia, orthostatic hypotension, hypotension, hypertension, bradycardia, acroedema, angina pectoris, extrasystoles, heart failure, erythema, myocardial infarction, cerebrovascular accident, myocardial ischemia, transient ischemic attack, atrial fibrillation, cardiac arrest, bundle branch block. Nervous system: paresthesia, migraine, hyperkinesia, vertigo, hypertonia, extrapyramidal dysfunction, involuntary muscle contraction, hypokinesia, neuralgia, dystonia, abnormal gait, hypoesthesia, ataxia, coordination disorder, hyperesthesia, ptosis, stupor. Endocrine system: hypothyroidism, goiter, gynecomastia. Digestive system: increased salivation, flatulence, gastritis, gastroenteritis, stomatitis, belching, hemorrhoids, dysphagia, molars, gingivitis, oesophagitis, enteritis, gastric ulcer, cholecystitis, cholelithiasis, duodenal ulcer, gastroesophageal reflux, glossitis, jaundice, diverticulitis, rectal bleeding, hiccough. “Systemic: flushing, rigors, alcohol intolerance, fainting, flu-like symptoms, hay fever.” Blood system: purpura, anemia, epistaxis, leukocytosis, leukopenia, lymphadenopathy, pulmonary embolism, granulocytopenia, lymphocytosis, lymphopenia, hypochromic anemia, coagulation dysfunction, gingival bleeding. “Metabolism and nutrition: weight loss, weight gain, elevated aminotransaminase, thirst, dry eyes, elevated alkaline phosphatase, impaired glucose tolerance, bilirubinemia, hypokalemia, obesity, hypoblood pool, hepatitis, and dehydration.” Musculoskeletal system: arthritis, muscle weakness, bone pain, bursitis, osteoporosis. “Mental system: decreased attention, amnesia, apathy, depression, increased appetite, increased depression, attempted suicide, confusion, increased libido, aggression, nightmares, drug dependence, depersonalization, hallucinations, euphoric feelings, mental depression, delusions, delusional, mood instability, panic, psychosis, catatonic disorder, depression.” “Female reproductive system: amenorrhea, galactorrhea, breast pain, breast enlargement, vaginal bleeding.” Respiratory system: cough, bronchitis, dyspnea, pneumonia, asthma, laryngitis, bronchospasm, phlegm. “Skin and accessories: rash, pruritus, photosensitivity, urticaria, acne, skin discoloration, eczema, alopecia, dermatitis, dry skin, ichthyosis, hirsutism, hypohidrosis, pigmentation, keratitis, cellulitis, pruritus anus.” Special sensations: adaptive accommodation disorder, taste reversal, tinnitus, conjunctivitis, eye pain, pupil dilation, photophobia, diplopia, abnormal tearing, cataract, loss of taste. Urinary system: polyuria, frequent urination, urinary retention, dysuria, facial edema, hematuria, oliguria, pyelonephritis, renal calculi, renal pain.
1. It is forbidden for those who are allergic to the active ingredient and/or any excipients in this product. Monoamine Oxidase Inhibitors (MAOIs) during the 14 days following the discontinuation of irreversible MAOI, patients receiving monoamine oxidase inhibitors (MAOIs), including selegiline at a daily dose of more than 10mg, should not take it concurrently. “Alternatively, it should not be given for a specified period of time after discontinuation of RIMA as specified in the reversible MAOI (RIMA) prescription.” MAOIs should not be given for the 14 days after discontinuation of this drug. 3. Concomitant use of linezolid is prohibited unless a device for close observation and monitoring of blood pressure is present, as described in the Drug Interactions section. 4. Concomitant medication with pimozide is prohibited, as described in Drug Interactions. 5. It is contraindicated in patients with known QT interval prolongation or congenital QT syndrome.
After marketing, there have been some spontaneous reports of adverse events after withdrawal, especially when the drug was stopped suddenly: 1. “Emotional irritability, irritability, agitation, dizziness, paresthesia (sensation of electric shock), anxiety, confusion, headache, laziness, mood instability, insomnia, hypomania, tinnitus, seizures, etc.” The above manifestations are generally self-limited, and severe withdrawal reactions have also been reported. Care should be taken to monitor patients for these possible withdrawal symptoms when the drug is discontinued. A gradual dose reduction is recommended to avoid abrupt discontinuation. If intolerable symptoms developed during dose reduction and discontinuation, a return to the previous treatment dose could be considered, with subsequent tapering at a slower rate. 2. Abnormal bleeding There have been reports of subcutaneous bleeding time and/or abnormal bleeding with SSRIs, for example, ecchymosis, gynecological bleeding, gastrointestinal bleeding, and other skin or mucosal bleeding. Caution is warranted in patients taking SSRIs, particularly in combination with active substances known to affect platelet function or other active substances that may increase the risk of bleeding, and in patients with a history of bleeding disorders. Hyponatremia is rarely reported with SSris. Hyponatremia may be caused by abnormal secretion of antidiuretic hormone (SIADH) and usually returns to normal upon discontinuation of treatment. Older female patients in particular may be at risk. 4. Akathisia/psychomotor restlessness The use of SSRIs/SNRIs has been linked to the formation of akathisia, characterized by subjective unpleasant or disturbing agitation, the need for constant movement, and the inability to sit and stand quietly. This is most likely to occur during the first few weeks of treatment. In patients suffering from these symptoms, increasing the dose may be harmful. 5. Patients with manic-manic depression may transition to manic episodes. It should be discontinued in patients who develop manic episodes. 6. Seizures Seizures are a potential risk when using antidepressants. The drug should be discontinued in patients with seizures. It should be avoided in patients with unstable epilepsy and carefully monitored in those whose seizures have been controlled. If the frequency of seizures increases, the drug should be discontinued. 7. Diabetes Mellitus In patients with diabetes mellitus, treatment with a certain SSRI may alter glycemic control. Adjustments in the dose of insulin and/or oral hypoglycemic agents may be required. 8. Clinical experience with simultaneous administration of SSRIs and ECT by electroconvulsive therapy is limited, and therefore, caution should be used. 9. St. John’s Wort Adverse reactions may be more common during the combined use of this product and herbal preparations containing St. John’s wort (St. John’s wort). Therefore, this product and St John’s wort preparations should not be taken simultaneously. 10. Psychiatric Disorders The treatment of patients with psychiatric disorders who have depressive episodes with this product may increase symptoms of psychiatric disorders. Medication should be taken under the guidance of a doctor. 11. Auxiliary materials (add this content according to the ingredients of each auxiliary material) this product
Load more

![[Byminle] 6s bring "music" home Byebye allergy](http://www.eng.venturepharma.cn/wp-content/uploads/2025/06/efbb73bdb88b4a2d8c2d5cf6ffea24ef-300x300.jpg)