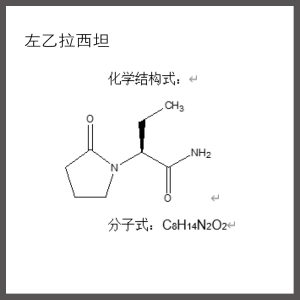
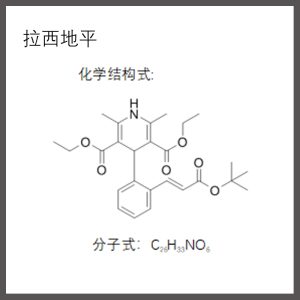
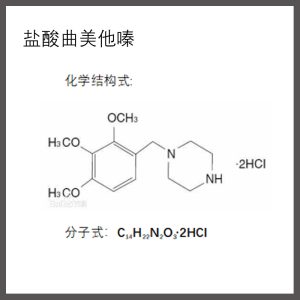
【 Drug name 】
Generic name: Adapalene gel
Product Name: Adapalene Gel (Bye beans)
Full Pinyin code: ADaPaLinNingJiao(WanQuan)
[Main ingredients] The active ingredient of this product is adapalene.
[Nature] This product is white or semi-white translucent gel.
[Indications/Indications] This product is suitable for the treatment of acne vulgaris characterized by acne, papules and pustules. It is also used to treat acne on the face, chest and back.
[Specification Model] 0.1%*15g
[Usage and dosage] Once a day, clean the affected area before going to bed and use it after drying. Apply a thin layer on the affected skin. In patients in whom the number of doses must be reduced or suspended, doses can be resumed when tolerance to adapalene has been confirmed. Do not use cosmetics that cause acne or are contractive.
[Adverse reactions] The most common adverse reactions in the first 2 to 4 weeks of treatment were erythema, dryness, scaling, itching, burning or tingling, which were mostly mild to moderate in degree. Less frequent adverse effects were sunburn, skin irritation, uncomfortable burning and stinging of the skin. The rare adverse reactions included acne, dermatitis and contact dermatitis, ocular edema, conjunctivitis, erythema, pruritus, skin discoloration, rash and eczema. If adverse reactions are serious, the frequency of medication should be reduced or the drug should be stopped.
Contraindication: Contraindication to adapalene or any component of the gel vehicle.
[Precautions] 1. If allergic or severe irritation occurs, the drug should be stopped. After determining the degree of local stimulation response, patients can reduce the frequency of medication, temporarily stop or completely stop the medication under the guidance of their doctors. 2. During the use of this product, if you are exposed to sunlight, including the sun lamp emitting ultraviolet rays, the dose should be reduced to the minimum. For patients who are often exposed to strong sunlight and who are allergic to sunlight themselves, special attention should be paid when exercising outdoors. Sun protection products and clothing are recommended on the treated area when exposure to the sun is unavoidable; When the climate is extremely abnormal, such as wind or cold, it may also produce irritation to patients receiving this product. 3. Avoid contact with the mucosa of eyes, lips, mouth, nose, inner canthus and other mucosal tissues. This product should not be used on the skin of cutting, abrasion, eczema or sunburn, and should not be used on the skin of patients with severe acne or eczema. This treatment should be avoided when other retinoic acid drugs or “waxy” hair removal methods are used. Please read the instructions carefully and follow the instructions.
In children, safety and efficacy have not been established in pediatric patients younger than 12 years of age.
In the clinical trials, participants were between the ages of 12 and 30 years, so it is not clear whether people older than 65 years differ from younger people.
[Use in pregnant and lactating women] There are no reports on the efficacy of this product in pregnant women. Davovan (adapalene gel) is not recommended during pregnancy. It is not known whether it is secreted in breast milk. As many drugs are secreted in breast milk, it is recommended that lactating women use this product with caution and do not apply it to the chest.
[Drug interactions] 1. At present, no interaction has been found between this product and other drugs that may be used in the skin at the same time. However, co-administration of other retinoids or other drugs with similar mechanisms of action should not be performed. 2. The chemical structure of adapalene is stable. It is not easy to decompose in air and sunlight. Extensive animal and human studies have not found phototoxicity and photosensitivity. However, the safety of adapalene in animals and humans during repeated exposures to sunlight or ultraviolet (UV) radiation is unknown. Excessive sunlight and ultraviolet radiation should be avoided when using this product. Adapalene has very low transdermal absorption, making interactions with systemic medications unlikely. There is no evidence that the efficacy of oral drugs such as contraceptives and antibiotics is affected by the use of this product in the skin.
[Drug overdose] This product can only be used on the skin, excessive application will not get faster or better efficacy, and can cause significant redness, desquamation or skin discomfort.
Adapalene, a retinoic acid compound, has been shown to have anti-inflammatory properties in both in vivo and in vitro inflammatory models. Adapalene has a stable chemical structure and does not easily decompose in air and light. “In terms of mechanism of action, adapalene, like retinoic acid, binds to specific retinoic acid nuclear receptors, but unlike retinoic acid, adapalene does not bind to protein-bound cytoplasmic receptors.” Adapalene has been shown to treat acne in skin drug tests using animal models established in mice, with activity in regulating cell differentiation and proliferation. The mechanism of action of adapalene is thought to reduce microacne formation by allowing hair follicle epithelial cells to differentiate normally. Adapalene was superior to retinoic acid in standard anti-inflammatory assays in vivo and in vitro. It can inhibit the chemotaxis of human multinucleated leukocytes and can inhibit the metabolism of multinucleated leukocytes by inhibiting the conversion of arachidonic acid to inflammatory mediators by lipid oxidation. This suggests that adapalene, when applied to acne lesions, can alleviate the inflammatory response mediated by cellular reactions. Studies in human clinical trials have shown that adapalene can alleviate the inflammatory reaction of acne, such as pustules and papules. The oral LD50 of this product in mice and rats is greater than 10ml/ kg, and long-term intake of this drug may cause some side effects associated with excessive oral vitamin A. Animal tests have shown a carcinogenic effect and a risk of increased skin cancer when exposed to sunlight. In vivo and in vitro studies showed no mutagenicity or genotoxicity. Adapalene was administered orally to rats at 20mg/ kg/day without reproductive toxicity. The teratogenic effects were observed when adapalene was administered to rats and rabbits at 24 and 48 times the maximum recommended total dose for human use, respectively.
[Pharmacokinetics] Adapalene has a very low transdermal absorption rate. In clinical trials, when the analytical sensitivity of adapalene was 0.15ng/ml, the plasma concentration of adapalene was too low to be detected. “The 14C-labeled adapalene, administered to rats (intravenous, intraperitoneal, oral, and dermal), rabbits (intravenous, oral, and dermal), and dogs (intravenous and oral), showed radioactivity in many tissues, with highest levels in the liver, spleen, adrenal gland, and ovary. Adapalene is metabolized in animals mainly through oxygen-demethylation, hydroxylation, and binding reactions.” It is excreted mainly through bile.
To store in a dark, sealed place
[Package] Medicinal hose, 15g/ cartridge/box.
【 Effective period 】 24 months
[Approval No.] H20066621
[Manufacturer] Hainan Quanxing Pharmaceutical Co., LTD

![[Bai Dou Campus Ambassador] Eliminate acne without leaving marks to prevent crow's feet](http://www.eng.venturepharma.cn/wp-content/uploads/2025/06/06de652d1805484d98c1082092c17b22.jpg)